Members
Publications
2022
-
Tetrahedron Chem, 2022, 2, 100018.
Valencia, E.; Poyatos, M.; Peris, E.
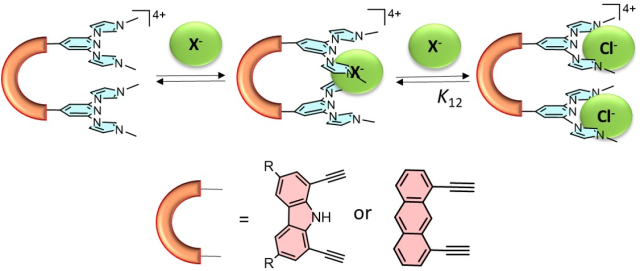
‘Pincer-tweezer’ tetraimidazolium salts as hosts for halides.
Article page: https://www.tetrahedron-chem.com/article/S2666-951X(22)00014-6/fulltext
-
Molecules, 2022, 27, 3699.
Ibáñez, S.
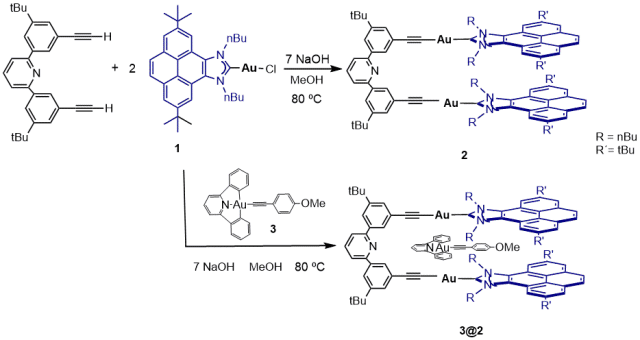
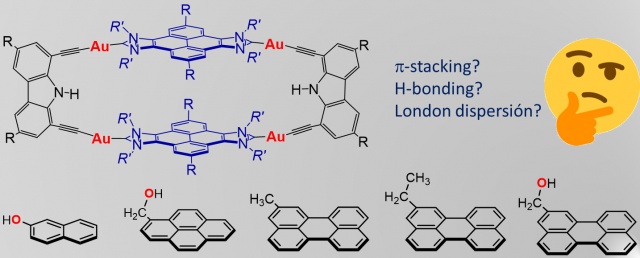
The New Di-Gold Metallotweezer Based on an Alkynylpyridine System.
Article page: https://www.mdpi.com/1420-3049/27/12/3699
-
Angewandte Chemie International Edition, 2022, 61, e202112513.
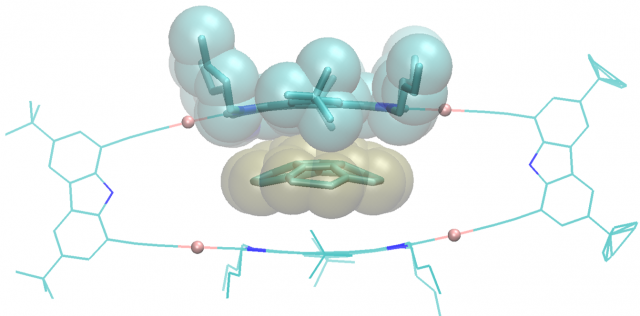
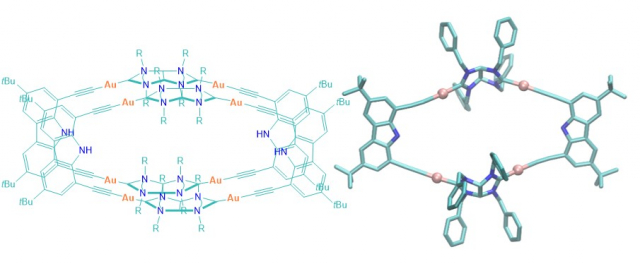
Ibáñez, S.; Vicent, C.; Peris, E.
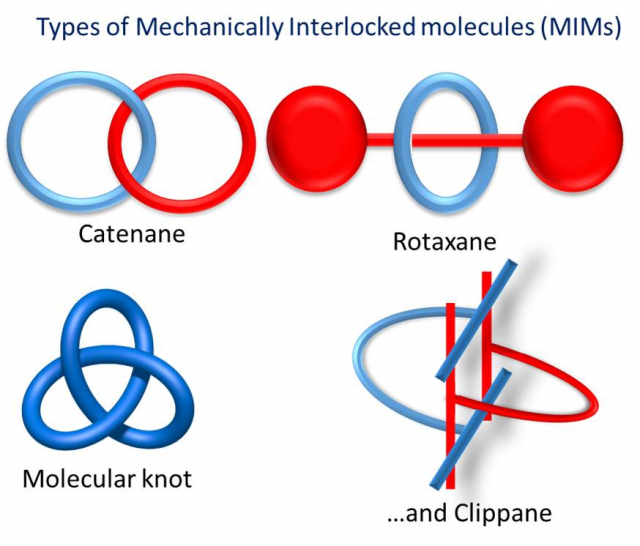
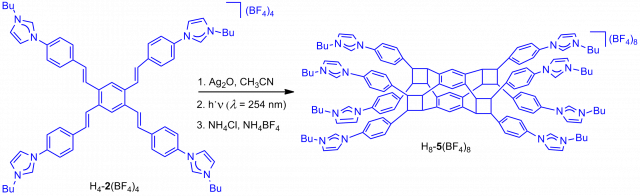
Clippane: A Mechanically Interlocked Molecule (MIM) Based on Molecular Tweezers.
Article page: https://doi.org/10.1002/anie.202112513
2021
-
ACS Catalysis, 2021, 11, 15212−15222.
Gonell, S.; Assaf, E.A.; Lloret-Fillol, J.; Miller, A.J.M.
-
Angewandte Chemie International Edition, 2021, 60, 20003-20011.
Ruíz-Zambrana, C.; Gutierrez-Blanco, A.; Gonell, S.; Poyatos, M.; Peris, E.
Article page: https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202107973
-
Dalton Transactions, 2021, 50, 12748-12763 .
Poyatos, M.; Peris, E.
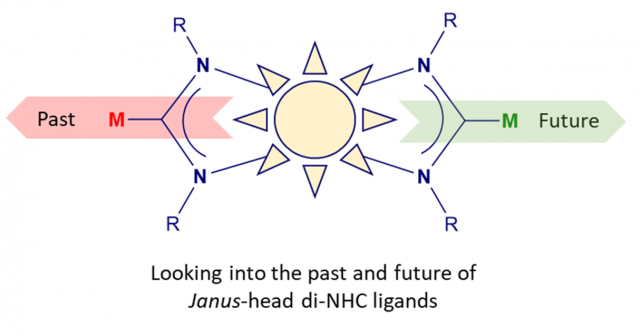
Insights into the past and future of Janus-di-N-heterocyclic carbenes.
Article page: https://pubs.rsc.org/en/content/articlelanding/2021/dt/d1dt02035h
-
European Journal of Inorganic Chemistry, 2021, 2434-2545.
Gutierrez-Blanco, A.; Dobbe, C.; Hepp, A.; Daniliuc, C.G.; Poyatos, M.; Hahn, E.; Peris, E.
Article page: https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/ejic.202100473
-
Angewandte Chemie International Edition, 2021, 60, 15412-15417.
Vicent, C.; Martínez-Agramunt, V.; Gandhi, V.; Larriba-Andaluz, C.; Gusev, D.G.; Peris, E.
Article page: https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202100914
-
Chemistry - A European Journal, 2021, 27, 9661-9665.
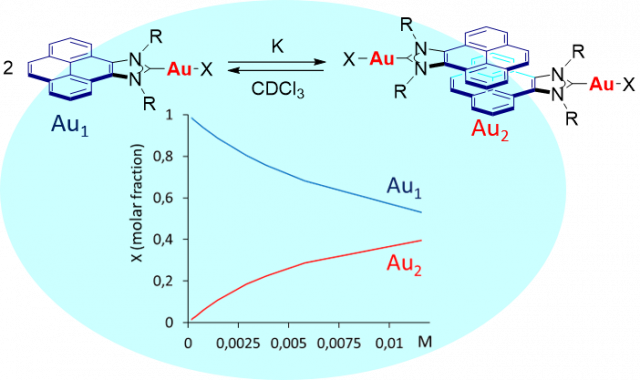
Ibáñez, S.; Peris, E.
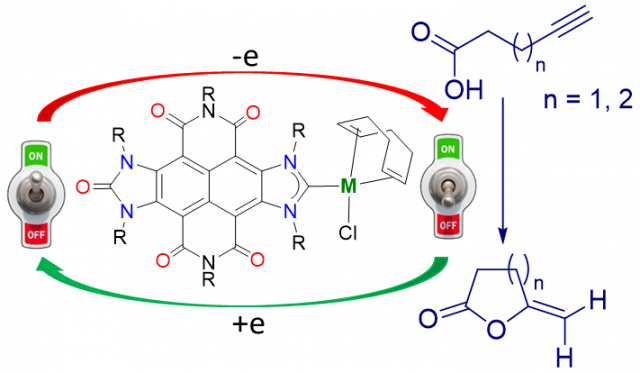
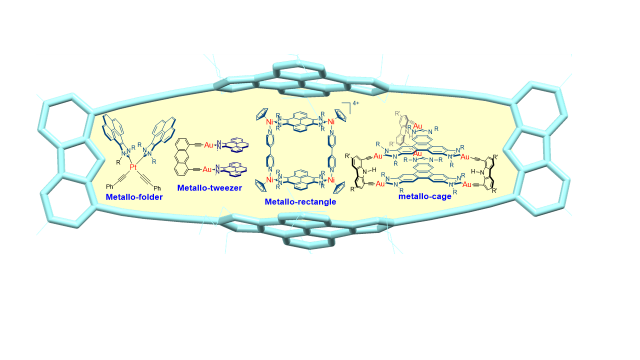
Shape-Adaptability and Redox-Switching Properties of a Di-Gold Metallotweezer.
Article page: https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.202100794
-
Sustainable Energy & Fuels, 2021, 5, 956-962.
Arcas, R.; Peris, E.; Mas-Marzá, E.; Fabregat-Santiago, F.
Article page: https://pubs.rsc.org/en/content/articlelanding/2021/SE/D0SE01322F#!divAbstract
2020
-
Organometallics, 2020, 39, 4078–4084.
Ibáñez, S.; Gussev, D.; Peris, E.
Article page: https://pubs.acs.org/doi/pdf/10.1021/acs.organomet.0c00639
-
Chemistry - A European Journal, 2020, 26, 11565-11570.
Dobbe, C.; Gutierrez-Blanco, A.; Tan, T.Tsai Yuan; Hepp, A.; Poyatos, M.; Peris, E.; Hahn, E.
Template-Controlled Synthesis of Polyimidazolium Salts by Multiple [2+2] Cycloaddition Reactions.
Article page: https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.202001515
-
Accounts of Chemical Research, 2020, 53, 1401–1413.
Ibáñez, S.; Poyatos, M.; Peris, E.
N-Heterocyclic Carbenes: A Door Open to Supramolecular Organometallic Chemistry.
Article page: https://pubs.acs.org/doi/10.1021/acs.accounts.0c00312
-
Journal of Organometallic Chemistry, 2020, 917, 121284.
Ibáñez, S.; Poyatos, M.; Peris, E.
Article page: https://www.sciencedirect.com/science/article/pii/S0022328X20301868
-
Nanoscale, 2020, 12, 14194-14203.
Hassanabadi, E.; Latifi, M.; Gualdrón-Reyes, A.F.; Masi, S.; Yoon, S.Joon; Poyatos, M.; Julián-López, B.; Mora-Seró, I.
Article page: https://pubs.rsc.org/en/content/articlehtml/2020/nr/d0nr03180a
-
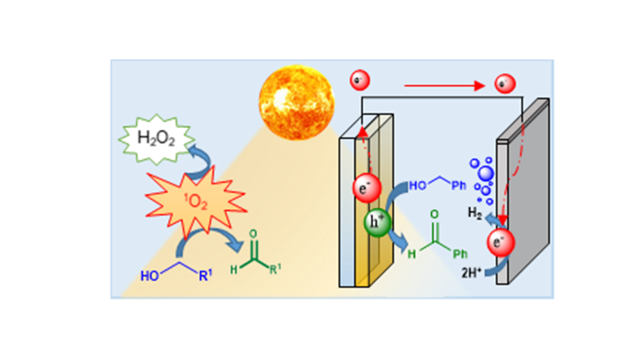
Angewandte Chemie International Edition, 2020, 59, 6860-6865.
Ibáñez, S.; Peris, E.
Article page: https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201915401
-
Dalton Transactions, 2020, 49, 3181-3186.
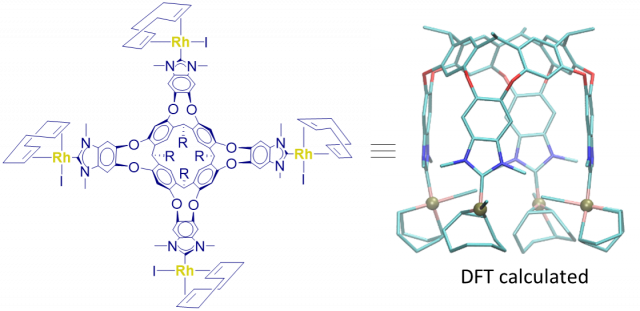
Ruiz-Botella, S.; Vidossich, P.; Ujaque, G.; Peris, E.
A resorcinarene-based tetrabenzoimidazolylidene complex of rhodium.
Article page: https://pubs.rsc.org/en/content/articlepdf/2020/dt/d0dt00060d
2019
-
Organometallics, 2019, 38, 4565-4569.
Gutierrez-Blanco, A.; Ibáñez, S.; Hahn, E.; Poyatos, M.; Peris, E.
A Twisted Tetragold Cyclophane from a Fused Bis-Imidazolindiylidene.
Article page: https://pubs.acs.org/doi/10.1021/acs.organomet.9b00719
-
Chemical Communications, 2019, 55, 14972-14975.
Martínez-Agramunt, V.; Peris, E.
Article page: https://pubs.rsc.org/en/Content/ArticleLanding/CC/2019/C9CC08595E#!divAbstract
-
European Journal of Inorganic Chemistry, 2019, 33, 3776-3781.
Gonell, S.; Peris, E.; Poyatos, M.
Article page: https://onlinelibrary.wiley.com/doi/full/10.1002/ejic.201900684